October 27, 2022
Blog
Webinar key learnings: How an integrated approach is crucial to success in the decentralized clinical trial landscape
Sarah Hrycyk
Marketing Programs Manager
Share
Last month, we hosted a webinar exploring how decentralization is the future of clinical trials. The discussion between Bob Bowdish, Loftware’s Director of Clinical Trials Sales for North America, and Maureen Perroni, Loftware’s Director of Content & Communications, examined how clinical trial labeling functions need to adapt to accommodate this changing landscape.
There has been growing interest in decentralized and hybrid models in the clinical trials landscape over the past decade, however this has accelerated significantly in recent years. Decentralized Clinical Trials (DCT) have proven their value and are becoming a permanent fixture of the industry and the shift away from centralized global distribution hubs is providing greater supply chain flexibility and resiliency.
As the clinical trial industry embraces decentralization, sponsors are demanding more and more, requiring partner organizations to be agile and adaptable in their approach. Due to accelerated decision-making and parallelized processes, changes to label data need to happen more frequently and at short notice, with the expectation that labels can then be produced on demand.
Consequently, a modern integrated labeling software solution is an essential tool for CROs, CMOs, and CDMOs, allowing them to eradicate manual processes which slow throughput and introduce risk into the process.
The decentralization agenda
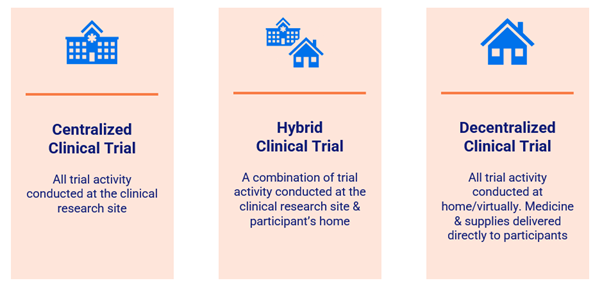
Decentralization is the key theme driving many of the transformative changes taking place across the clinical trials industry.
There are certain sectors of the industry that are more inclined to utilize decentralized clinical trials, such as biologics, personalized medicines, and orphan drugs which are not ideally suited to conventional trial models, centralized production, and patient administering.
These areas require flexibility in labeling to adjust to changes in protocol, site, or participant geography. If organizations continue to rely on manual processes and disparate systems, the labeling function can easily become an operational bottleneck.
Decentralized and hybrid trial models are also perceived as a solution to some of the recruitment and retention challenges that have been a persistent feature of the clinical trials landscape. This shift in approach should broaden and diversify the pool of available patients. By cutting travel time to trial sites or delivering packs directly to the home, the patient experience is improved, hopefully aiding with retention.
Considerations when it comes to labeling
One of the major challenges that can make labeling difficult is translation management; ensuring the efficient and accurate translation of master label text into country label text while meeting regulatory requirements. New country-specific labels can be quickly and easily created when using labeling software with an integrated library of approved translations and an automated system for verifying compliance with regulatory rules. This allows for the automation of many aspects of the labeling process, ensuring that approved and validated labels can be printed on-demand from anywhere in the world with access to the cloud labeling solution.
Ultimately, technology can be harnessed to streamline operations and speed up processes, positioning organizations as experts in label data management, design, and print.
Clinical trial organizations face a simple choice of either developing in-house labeling expertise or finding a partner who can. Sponsors are now specifically looking to work with organizations that can take on responsibility for labeling design and creation.
Implementing a labeling solution in a decentralized world
Organizations need software that offers end-to-end management of the labeling process that can integrate with other systems and minimize the need for human intervention. With sponsors becoming increasingly demanding, it is critical to avoid any inefficiency which could lead to costly errors and slow down the delivery of a study.
A modern integrated approach to labeling can aid in the agile supply chain demanded by sponsors, thereby providing the desired flexibility and scalability of manufacturing operations.
Adopting a cloud-based labeling platform, such as Loftware Prisym 360, CROs, CMOs and CDMOs can allow for a consistent environment across a distributed global supply chain with approved and validated labels able to be printed on-demand from anywhere in the world. Interoperability with ERP and MES (Manufacturing Execution Systems) allows for the transfer of critical label data and allows Prisym 360 to serve as a single source of truth for regulated label content.
Watch the webinar on-demand to discover how a clinical labeling solution holds the keys to success in the decentralized clinical trial landscape.

